In regard to antibody function identify the mismatched pair – In the realm of immunology, antibodies play a crucial role in recognizing and neutralizing foreign invaders. Understanding the structure and function of antibodies is essential for harnessing their potential in research, diagnostics, and therapeutics. This article delves into the concept of antibody-antigen interactions, exploring the intricacies of how antibodies bind to specific antigens.
Furthermore, it highlights the significance of identifying mismatched antibody-antigen pairs and discusses the potential consequences of their use.
Antibody Structure and Function
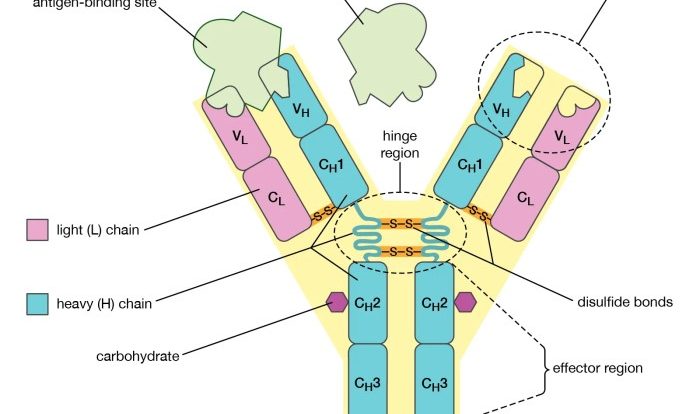
Antibodies are specialized proteins produced by the immune system in response to foreign substances or pathogens. They play a crucial role in recognizing and neutralizing antigens, triggering immune responses, and protecting the body from infections.
The basic structure of an antibody consists of four polypeptide chains: two heavy chains and two light chains. These chains are linked together by disulfide bonds to form a Y-shaped molecule.
The variable region of an antibody, located at the tips of the arms of the Y, is responsible for binding to specific antigens. Each antibody has a unique variable region that allows it to recognize and bind to a specific antigen.
The constant region, located at the base of the Y, determines the antibody’s class and effector functions.
There are five main classes of antibodies: IgG, IgM, IgA, IgD, and IgE. Each class has a different role in the immune response. IgG is the most abundant antibody class and is responsible for long-term immunity. IgM is the first antibody produced in response to an infection and is involved in early immune responses.
IgA is found in mucosal secretions and protects against infections at mucosal surfaces. IgD is expressed on the surface of B cells and is involved in B cell activation. IgE is involved in allergic reactions.
Antibody-Antigen Interactions

Antibodies recognize and bind to antigens through a process called antigen-antibody binding. The variable region of the antibody binds to a specific epitope on the antigen. The epitope is a small, specific region of the antigen that is recognized by the antibody.
There are different types of antibody-antigen interactions, including:
- Direct binding: The antibody binds directly to the antigen without the need for any other molecules.
- Indirect binding: The antibody binds to the antigen through a bridge molecule, such as a complement protein.
The affinity of an antibody for an antigen is a measure of how strongly the antibody binds to the antigen. The avidity of an antibody for an antigen is a measure of the overall strength of the antibody-antigen interaction, taking into account the number of binding sites and the affinity of each binding site.
Mismatched Antibody-Antigen Pairs
A mismatched antibody-antigen pair is a pair of antibody and antigen that do not bind to each other. This can occur for a number of reasons, including:
- The antibody is not specific for the antigen.
- The antigen has changed its structure, so that it is no longer recognized by the antibody.
- The antibody is not in the correct conformation to bind to the antigen.
Using a mismatched antibody-antigen pair can have a number of consequences, including:
- The antibody will not be able to neutralize the antigen, which can lead to infection or disease.
- The antibody may cross-react with other antigens, which can lead to false-positive results in diagnostic tests.
Applications of Antibodies
Antibodies are used in a wide variety of research and diagnostic applications, including:
- Immunoassays: Antibodies are used in immunoassays to detect and quantify antigens in samples. This is a common technique used in clinical diagnostics, research, and food safety testing.
- Flow cytometry: Antibodies are used in flow cytometry to identify and characterize cells. This technique is used in immunology, cancer research, and drug discovery.
- Immunohistochemistry: Antibodies are used in immunohistochemistry to localize antigens in tissues. This technique is used in pathology and research to study the expression and localization of proteins in cells and tissues.
Antibodies are also being developed for therapeutic applications, such as:
- Cancer therapy: Antibodies are being developed to target and kill cancer cells.
- Autoimmune diseases: Antibodies are being developed to suppress the immune system in autoimmune diseases.
- Infectious diseases: Antibodies are being developed to protect against and treat infectious diseases.
However, there are challenges and limitations to using antibodies in clinical settings, including:
- Immunogenicity: Antibodies can be immunogenic, meaning that they can trigger an immune response in the patient. This can lead to side effects, such as allergic reactions and anaphylaxis.
- Cost: Antibodies can be expensive to produce and administer.
- Efficacy: Antibodies may not be effective in all patients, and their efficacy can be affected by factors such as the patient’s immune status and the target antigen.
Detailed FAQs: In Regard To Antibody Function Identify The Mismatched Pair
What is the role of antibodies in the immune system?
Antibodies are specialized proteins that recognize and neutralize foreign substances, such as bacteria, viruses, and toxins, by binding to specific antigens on their surface.
How do antibodies recognize antigens?
Antibodies have variable regions that can bind to specific antigens with high affinity. This binding is highly specific and allows antibodies to distinguish between different antigens.
What are the consequences of using mismatched antibody-antigen pairs?
Using mismatched antibody-antigen pairs can lead to reduced binding affinity, impaired neutralization, and potential immune dysregulation.