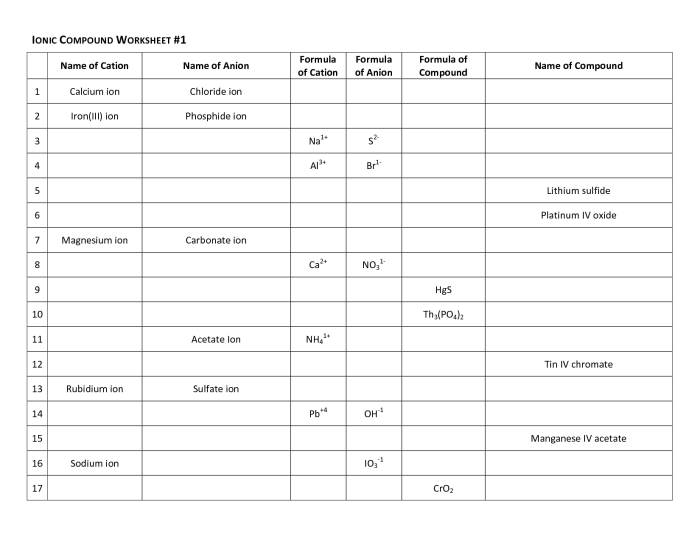
Ionic compound worksheet answer key presents a comprehensive overview of ionic compounds, encompassing their formation, properties, naming conventions, formula writing, and applications. This guide serves as an invaluable resource for students seeking to enhance their understanding of this fundamental chemical concept.
Ionic compounds, characterized by the electrostatic attraction between positively charged cations and negatively charged anions, exhibit unique physical and chemical properties. This guide delves into the intricacies of ionic compound formation, providing numerous examples and exploring their diverse applications in various fields.
Ionic Compounds
Ionic compounds are chemical compounds composed of ions, which are atoms or molecules that have lost or gained electrons, resulting in a net electric charge. The oppositely charged ions are attracted to each other by electrostatic forces, forming an ionic bond.
Ionic compounds are typically formed between a metal and a nonmetal. The metal atom loses one or more electrons to achieve a stable electron configuration, becoming a positively charged ion (cation). The nonmetal atom gains the electrons lost by the metal atom, becoming a negatively charged ion (anion).
The charges of the ions are balanced, resulting in an overall neutral compound.
Examples of Ionic Compounds
- Sodium chloride (NaCl): Sodium atom loses one electron to chlorine atom, forming Na+ and Cl- ions.
- Potassium oxide (K2O): Potassium atom loses one electron to oxygen atom, forming K+ and O2- ions.
- Calcium fluoride (CaF2): Calcium atom loses two electrons to fluorine atom, forming Ca2+ and F- ions.
Physical and Chemical Properties of Ionic Compounds
- Physical Properties:
- Ionic compounds are typically solids at room temperature.
- They have high melting and boiling points due to the strong electrostatic forces between the ions.
- They are generally soluble in polar solvents like water.
- Chemical Properties:
- Ionic compounds undergo ionic reactions, where ions exchange places to form new compounds.
- They react with acids and bases to form salts.
- They can be decomposed by electrolysis, where an electric current separates the ions.
Naming Ionic Compounds
Ionic compounds are named using the Stock system, which assigns a Roman numeral to the metal ion to indicate its charge. The name of the metal is then followed by the name of the nonmetal, which ends in -ide.
Binary Ionic Compounds
Binary ionic compounds are composed of two elements, a metal and a nonmetal. The metal is named first, followed by the nonmetal. The name of the nonmetal ends in -ide.
For example, the ionic compound NaCl is named sodium chloride. The metal is sodium (Na), and the nonmetal is chlorine (Cl).
Polyatomic Ionic Compounds
Polyatomic ionic compounds are composed of a metal and a polyatomic ion. A polyatomic ion is a group of atoms that has an overall charge. The name of the polyatomic ion is typically written in parentheses, and the charge of the ion is indicated by a Roman numeral.
For example, the ionic compound CaCO 3is named calcium carbonate. The metal is calcium (Ca), and the polyatomic ion is carbonate (CO 32-).
Exceptions and Special Cases
There are a few exceptions and special cases to the rules for naming ionic compounds.
- Some metals can form ions with variable charges. In these cases, the charge of the ion is indicated by a Roman numeral in parentheses.
- Some polyatomic ions have multiple names. For example, the hydroxide ion (OH –) can also be called the hydroxyl ion.
- Some ionic compounds have common names that are still used today. For example, water (H 2O) is a common name for the ionic compound dihydrogen oxide.
Writing Ionic Compound Formulas
Writing ionic compound formulas involves a systematic approach to ensure the correct representation of the chemical composition of ionic compounds. This process considers the charges of the ions involved and the need to maintain electrical neutrality in the compound.
Steps in Writing Ionic Compound Formulas
- Identify the cation and anion involved in the compound.
- Determine the charges of the ions using the periodic table or the rules for common ions.
- Balance the charges of the ions by adjusting the subscripts of the ions.
- Write the formula using the balanced subscripts and the chemical symbols of the ions.
Examples of Writing Ionic Compound Formulas
Binary Ions
* Sodium chloride (NaCl): Na+ (charge +1) and Cl- (charge1)
-
Calcium oxide (CaO)
Ca2+ (charge +2) and O2- (charge
- 2)
Polyatomic Ions
* Ammonium sulfate ((NH4)2SO4): NH4+ (charge +1) and SO42- (charge2)
-
Potassium nitrate (KNO3)
K+ (charge +1) and NO3- (charge
- 1)
Exceptions and Special Cases
* Variable charge metal ions:Some metal ions can have multiple charges. In such cases, the charge of the ion must be specified in the name of the compound, e.g., iron(II) oxide (FeO) vs. iron(III) oxide (Fe2O3).
Polyatomic ions with variable charges
Some polyatomic ions can also have variable charges. The charge of the ion must be specified in the name of the compound, e.g., hydrogen carbonate (HCO3-) vs. carbonate (CO32-).
Balancing Ionic Equations: Ionic Compound Worksheet Answer Key
Balancing ionic equations involves adjusting the coefficients of reactants and products to ensure that the number of atoms of each element is equal on both sides of the equation. This is essential for maintaining the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Balancing Ionic Equations in Acidic Solutions
In acidic solutions, hydrogen ions (H+) are present, and the following steps can be used to balance the equation:
- Write the unbalanced ionic equation.
- Balance the charge on both sides of the equation by adding electrons (e-) to one side or the other.
- Add H+ ions to the side that is deficient in hydrogen.
- Balance the oxygen atoms by adding H2O molecules.
- Balance the remaining elements by adjusting the coefficients of the reactants and products.
Balancing Ionic Equations in Basic Solutions
In basic solutions, hydroxide ions (OH-) are present, and the following steps can be used to balance the equation:
- Write the unbalanced ionic equation.
- Balance the charge on both sides of the equation by adding electrons (e-) to one side or the other.
- Add OH- ions to the side that is deficient in hydroxide.
- Balance the oxygen atoms by adding H2O molecules.
- Balance the remaining elements by adjusting the coefficients of the reactants and products.
Balancing Ionic Equations in Neutral Solutions, Ionic compound worksheet answer key
In neutral solutions, neither H+ nor OH- ions are present, and the following steps can be used to balance the equation:
- Write the unbalanced ionic equation.
- Balance the charge on both sides of the equation by adding electrons (e-) to one side or the other.
- Balance the remaining elements by adjusting the coefficients of the reactants and products.
Special Cases and Exceptions
There are some special cases and exceptions to consider when balancing ionic equations:
- Polyprotic acids: Acids that can donate more than one H+ ion require special balancing techniques.
- Redox reactions: Reactions involving electron transfer require balancing the number of electrons transferred.
- Precipitation reactions: Reactions that form an insoluble solid require careful balancing of the ions involved.
Applications of Ionic Compounds
Ionic compounds have numerous applications in various fields, including everyday life, industry, commerce, and the environment. They play crucial roles in diverse processes and products, ranging from household items to industrial chemicals.
Everyday Life
*
-*Table salt (NaCl)
Essential for seasoning and preserving food.
-
-*Baking soda (NaHCO3)
Used as a leavening agent in baking and as a cleaning agent.
-*Toothpaste (CaF2)
Strengthens teeth and prevents cavities.
-*Antiperspirants (AlCl3)
Block sweat glands, reducing perspiration.
Industry and Commerce
*
-*Fertilizers (NH4NO3, KCl)
Provide essential nutrients for plant growth.
-
-*Batteries (Li-ion batteries)
Power electronic devices such as laptops and cell phones.
-*Construction materials (CaCO3, CaSO4)
Used in cement, plaster, and drywall.
-*Water treatment (FeCl3, Al2(SO4)3)
Coagulate impurities, purifying water.
Environmental and Biological Significance
*
-*Ocean salinity (NaCl)
Maintains the Earth’s water balance and supports marine life.
-
-*Electrolytes in living organisms (Na+, K+, Cl-)
Regulate fluid balance and nerve function.
-*Calcium in bones and teeth (CaCO3)
Provides structural support and strength.
-*Potassium in plants (K+)
Essential for photosynthesis and growth.
Ionic compounds are indispensable in our daily lives, industrial processes, and the functioning of the natural world. Their unique properties and versatility make them invaluable for a wide range of applications, contributing to human health, technological advancements, and environmental sustainability.
FAQ Overview
What are ionic compounds?
Ionic compounds are chemical compounds composed of positively charged cations and negatively charged anions, held together by electrostatic forces.
How are ionic compounds named?
Ionic compounds are named using the Stock system, where the cation is named first, followed by the anion. The charge of the cation is indicated using Roman numerals.
How are ionic compound formulas written?
Ionic compound formulas are written by balancing the charges of the cation and anion. The subscripts in the formula indicate the number of atoms of each element required to achieve charge neutrality.
What are some applications of ionic compounds?
Ionic compounds have a wide range of applications, including as electrolytes in batteries, as fertilizers in agriculture, and as pigments in paints.